Contributing Editors: J. Boen and A. Breckenridge
Scaling biomanufacturing for Industrial Biology
One of the unique challenges for Industrial Biology start-ups is scaling biomanufacturing. While there is some industry and business know-how, most of it is resident in the large industrial players. A large number of start-up founders tend to be young Ph.D. students who are brilliant in the labs but have limited exposure to industrial-scale production. In addition, biomanufacturing itself is still in its early stages of development, where it lacks manufacturing standards and the availability of market tools to help guide start-ups to overcome scaling.
Due to these challenges, many companies have to build their capabilities from scratch via determination and trial and error. Making it a time consuming and capital intensive effort that has the potential to derail a young company that’s unable to survive. To reduce the risk and accelerate timing, there are some areas that start-ups should consider:
- Focus on lean manufacturing: start with the end in mind (Sean and Gaurab from Solugen speak about this route to scale in this talk)
- Implementation of process controls during scaling to understand and manage risks
- Work with and engage experienced industrial talent early (have a co-founder or early hire who has worked in and understands commercial scale operations to help drive manufacturing development)
Incorporate lean manufacturing principles
Lean manufacturing principles that Japanese manufacturers first introduced in the early 80’s (summarized in the book, Machine That Changed the World ¹), helped reduce lead time for developing new car models and are well understood in traditional and current manufacturing. Integrating this lean approach to manufacturing, design, and supply chains allowed the manufacturers to adjust their strategy early in the development process to account for supplier capabilities and manufacturing scalability risk. Incorporating these considerations and boundary conditions for biomanufacturing will help start-ups eliminate options/paths that are not economically viable early. This is one of the key causes of the “valley of death” in technology commercialization. Additionally, lab-scale processes should be modeled after or as closely as possible to full-scale deployment. Enabling a glimpse into how the technology will perform under commercial conditions will allow for early adjustments to the process. This will not only save industrial biology companies time when they reach scale, but it will substantially reduce the amount of CapEx needed to adjust the process.
Amyris, a leader in the field of Industrial Biology, has publicly discussed its strategy for scaling production processes. Simply put, Amyris starts by keeping commercial scale in mind from the early stages of technology development. This has allowed them to increase their odds of success and reduce potential capital losses when a technology is finally brought up to commercial levels of production. Below is a figure from a 2020 paper² released by Amyris, highlighting the accuracy its early-stage fermentation performance data has had in correlation with performance data when the process was scaled to a 100,000 L fermenter.
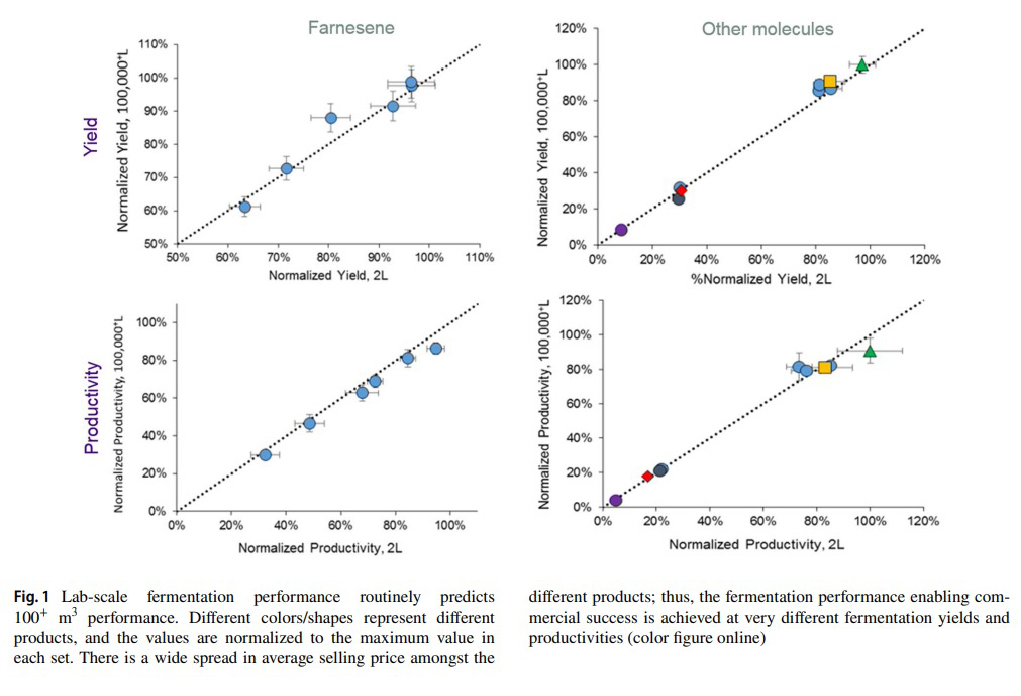
“Implementing the techniques for scaling down full-scale process conditions to the lab or pilot scale is a critical aspect of a successful technology transfer approach.” – Amyris
Implement statistical control processes
In the aforementioned 2020 paper, leaders in process development at Amyris discussed the importance of process controls during scale-up efforts. The authors said, “Excellence in the technical aspects of scale-up is insufficient to minimize risk of technology transfer failure. Many technology transfers struggle to succeed initially, not because the chemical engineering principles were poorly understood and applied, but because of the unexpected (e.g., the wrong hydration state of a critical raw material was ordered, or an unmonitored incubator could not consistently maintain the correct temperature). More generally, an important aspect of the process or execution was not well communicated, not double-checked, or simply not under control. Having modalities to manage and monitor industrial production is crucial to success. Being able to quickly identify failure points and course correction is necessary to ensure the right process control and quality of the overall procedure. Nowadays, industrial biology companies operating at a commercial scale often embed software tools for data collection to track and optimize processes to improve manufacturing productivity. In concert with analytics tools, principals need to be put in place to act as a stop/go barrier for decision-makers controlling the commercial manufacturing process. Establishing clearly defined procedures and crafting a strategy for the next steps to take when a failure occurs is a necessity for process engineering teams in biomanufacturing to mitigate both chronological and financial losses. Embodying an almost militant approach to the process control levers will lead to more opportunities to take shots on goal, increasing the likelihood of obtaining a commercially successful production process.
Recruit industrial talent/experts early
Producing anything at scale requires a completely different set of skills than what’s needed to demonstrate something is effective at smaller capacities. Yet this is a core requirement for successful technology transfer. A viable economic model at scale will determine the ultimate success of the start-up. This makes having access to the necessary knowledge and experience of bringing a product from lab to commercial scale essential. This ties back to our conversation earlier about designing for scale from an early stage. Having a symbiotic balance between technical and manufacturing knowledge will be key in getting industrial biology companies to effectively produce bioproducts at scale. Which is, of course, the dream of all young startups. Incorporating talents from both sides of the coin will de-risk business success and allow for efficient capital allocation.
What to expect next: Investment for biomanufacturing infrastructure development
With this in mind, we must begin the discussion around what’s needed for the Industrial Biology industry to accelerate to mass adoption. Not just in consumer products, but in every facet of the economy that biology has the potential to disrupt. Achieving a goal of this magnitude will only be attainable once the biomanufacturing infrastructure is developed to allow for commercial-scale production of bioproducts. In our next blog, we’ll discuss the necessity for reaching this milestone and the current state for government and private investment interests in this area.
References:
- Machine That Changed the World synopsis: (https://dl1.cuni.cz/pluginfile.php/218097/mod_resource/content/0/womack_the_machine_that_changed_the_world.PDF)
- Clean manufacturing powered by biology: how Amyris has deployed technology and aims to do it better: (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7695652/pdf/10295_2020_Article_2314.pdf)


Recent Comments